We Aid Compliance
MDR, UDI and IVDR

MDR, UDI and IVDR COMPLIANCE LABELLING SOLUTIONS
At IMS Labels we understand that your MedTech innovation is not complete with just the product itself but also the precision in how it is presented to the world.
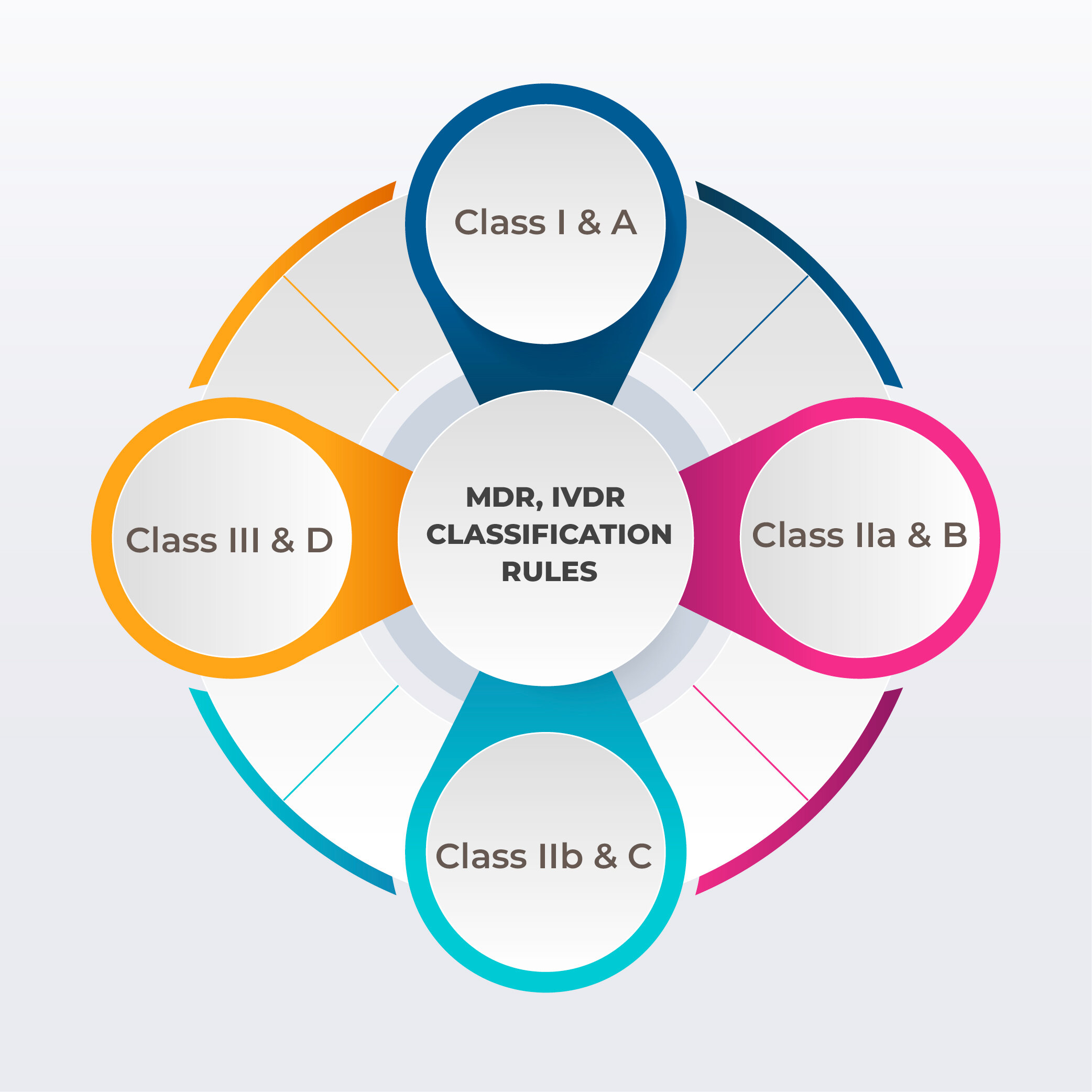
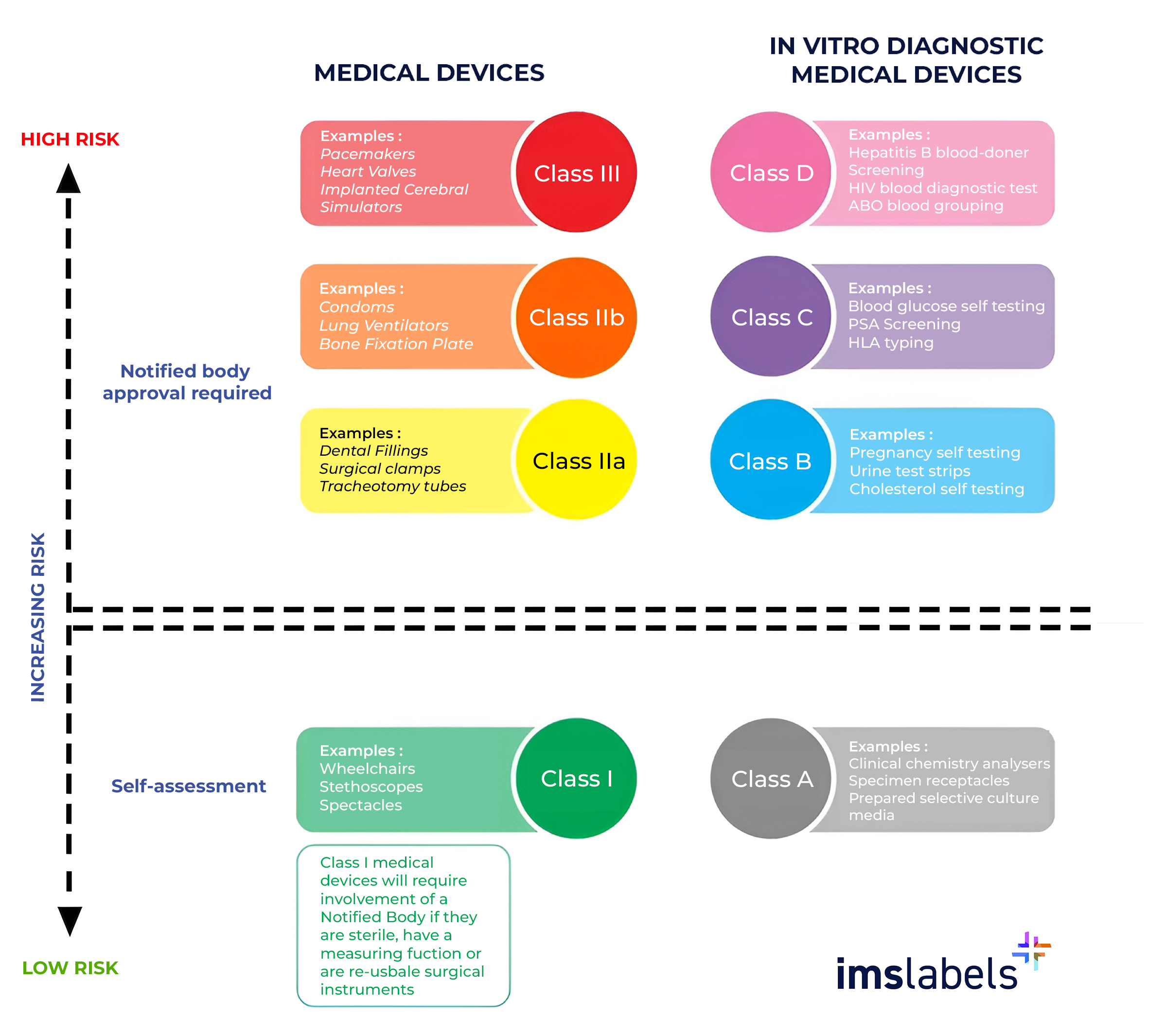
MDR (Medical Device Regulation) and IVDR (In Vitro Diagnostic Medical Devices Regulation) are the overarching regulations that establish the legal framework for medical devices and in vitro diagnostic medical devices in the EU. These standards ushered in a new era of regulation.
GET A FREE EXPERT CONSULTATION
Contact us for a brief demonstration on how you can get a head start on MDR, UDI and IVDR compliance.

MDR mandates the implementation of a Unique Device Identification (UDI) system for medical devices, enhancing traceability and transparency throughout the supply chain.
IVDR requires manufacturers to label IVD products with manufacturer details, product and batch identification, expiry, storage conditions, usage warnings, and performance metrics. This data must match the member state's official languages.
Our comprehensive suite of MDR, UDI and IVDR solutions are more than just labels.
WE OFFER AN END-TO-END SOLUTION FOR YOU THAT INCLUDES :
- Multiply, Booklet, Leaflet Labels
- High-quality Print Consumables
- Industry-Leading-Printer Hardware
- Intuitive Print Software
- Seamless Integration With Your ERP System
With an in-depth grasp of the regulatory landscape, we tailor our offerings to fit the unique needs of your business, ensuring you stay ahead of the curve. Whether you are navigating through the complexities of timely MDR, UDI or IVDR compliance, or striving for seamless data management, our solutions are crafted with precision to ensure compliance is not a hurdle.
GET A FREE EXPERT CONSULTATION
Contact us for a brief demonstration on how you can get a head start on MDR, UDI and IVDR compliance.

